
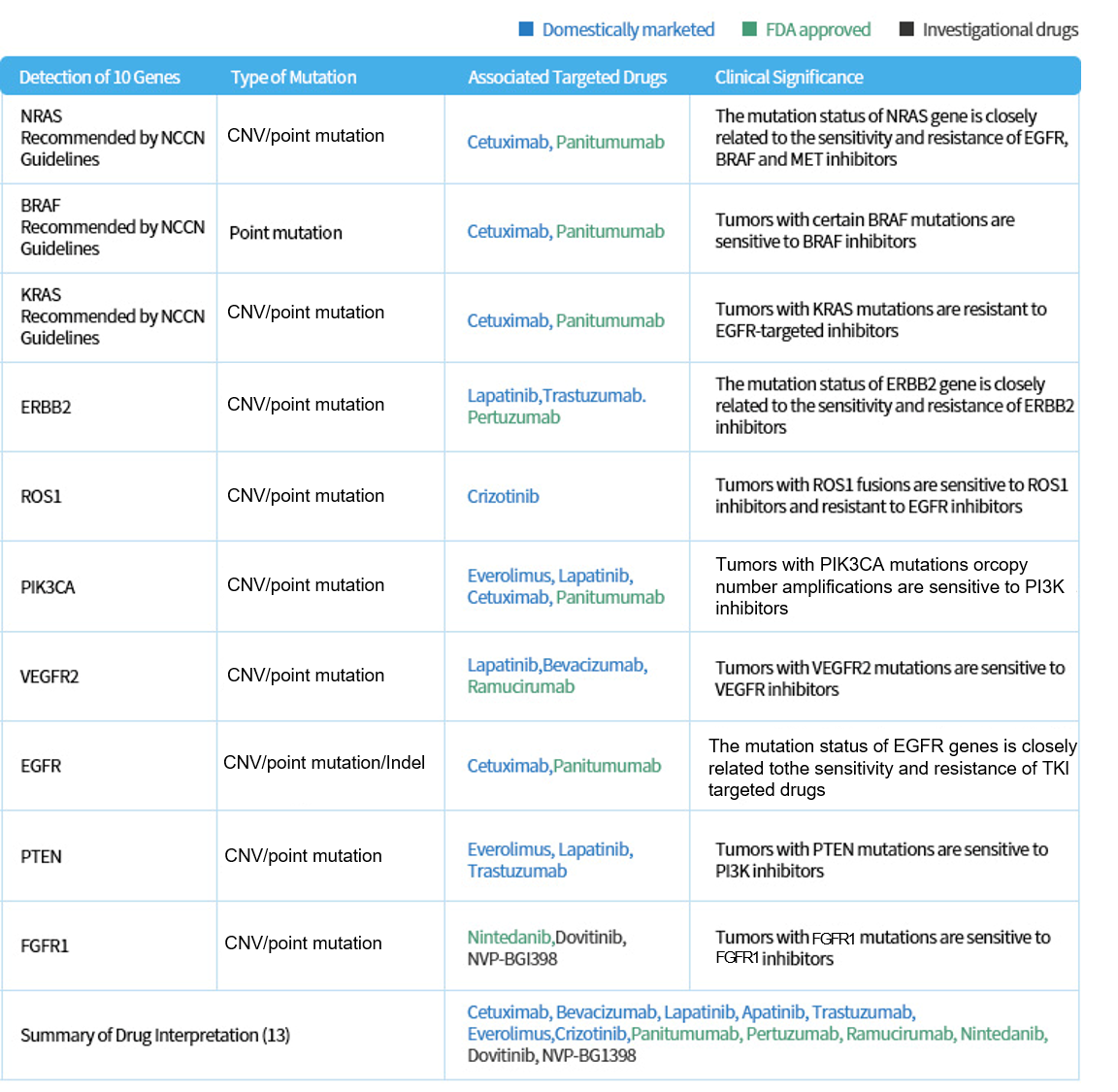
The sensitivity and toxic and side effects of 51 targeted drugs (including 34 approved drugs and 17 investigated drugs), as well as 9 commonly used chemotherapy drugs in colorectal cancer treatment regimens are precisely and accurately interpreted, which assist clinicians to formulate the personalized treatment regimens more accurately, and improve the survival benefit of patients.
It enables:
(1) Comprehensively include key genes of colorectal cancer, break through the limitations of conventional methods;
(2) Assist in the selection of appropriate drugs and treatment regimens, and improve treatment effects;
(3) Perform dynamic monitoring.
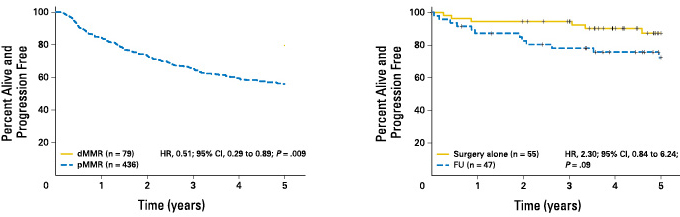
Stage II colorectal cancer patients with MSI-H have a better prognosis, and the 5-year survival rate is significantly higher than that with MSI-L/MSS[1].
Stage II colorectal cancer patients with MSI-H do not benefit from fluorouracil chemotherapy[1]
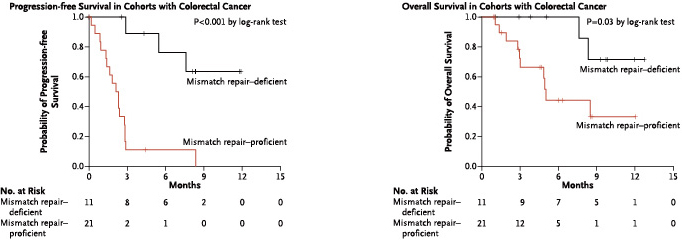
[1] Sargent DJ, et al.Defective Mismatch Repair As a Predictive Marker for Lack of Efficacy of Fluorouracil-Based Adjuvant Therapy in Colon Cancer. J ClinOncol.2010 Jul 10;28(20):3219-26.
[2] Le DT, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520.

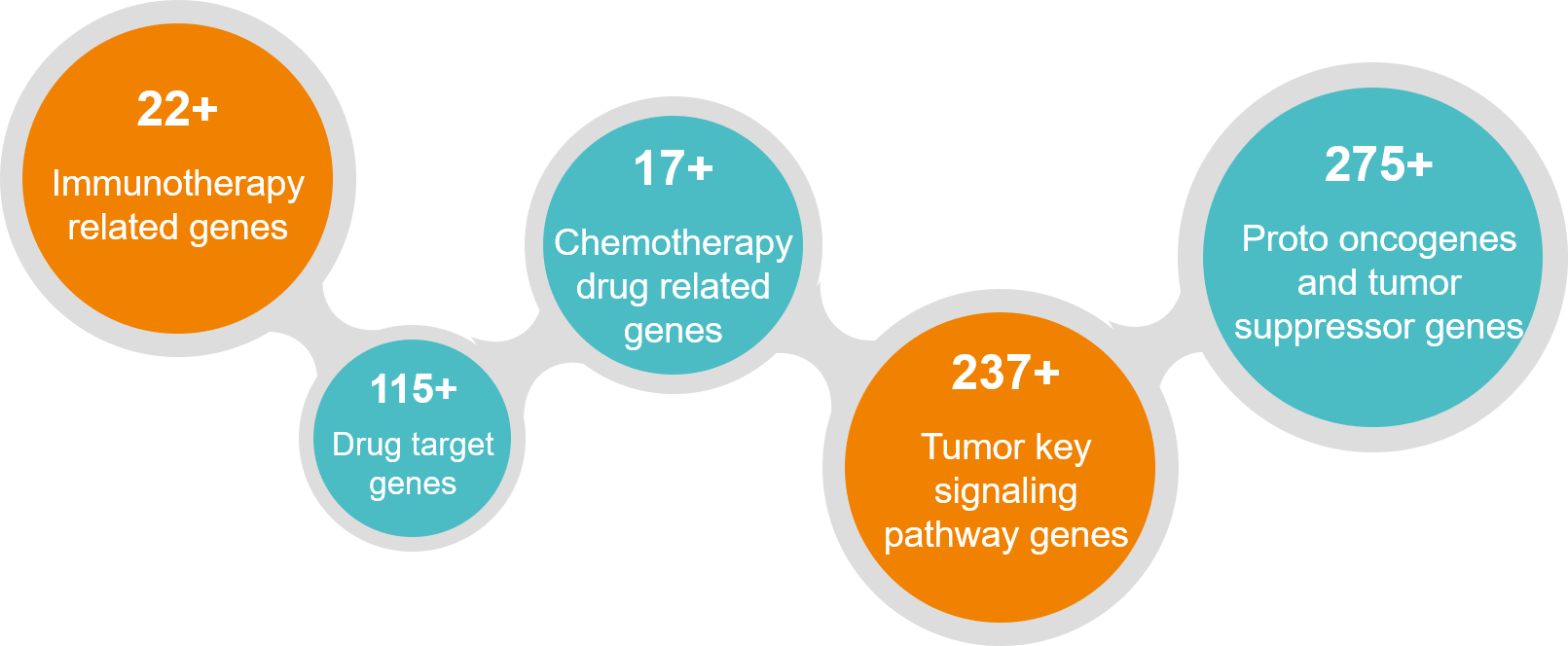
- Detection plan of 22 genes related to colorectal cancer: patients who are preliminarily diagnosed with colorectal cancer and require targeted drugs;
Detection plan of 868 genes related to colorectal cancer: patients with colorectal cancer who have progressed or recurred after multiple lines of treatment and require more treatment options;
Patients with colorectal cancer who seek for the optimal treatment regimen;
Patients with stage IIIB and IV colorectal cancer whose tissue is not available (or tissue has been stored for more than 1 year) and who meet the above criteria, ctDNA testing is an option.


